Evaluation of anti-diarrhoeal activity of Bacillus coagulans MTCC 5856 and its effect on gastrointestinal motility in Wistar rats
Int J Pharm Bio Sci. 2016;1:311–16
Diarrhoea is a condition, wherein frequency of bowel movements and/or looseness of stool will be more than the normal. It is an imbalance between the secretory and absorptive processes in the intestines. It has been reported that in several developing countries, ~1.7 billion diarrhoeal cases occur every year, of which 760,000 children (≤5 years) are dying each year. Although several anti-diarrhoeal agents of synthetic base are in use, quest for alternatives that are safe and effective is on-going given that synthetic drugs are associated with unwanted side effects. Probiotics are one such alternative which have traditionally been part of our diet in the form of dairy products (yoghurts or fermented drinks) or in lyophilised form. Probiotics are live microbial feed additions that are known to improve human and/or animal health. Probiotics have gained importance in the last couple of decades or so, due to their ability to improve the composition of the gastrointestinal microbiota and resultant diminished risk of related disorders. Several studies have also demonstrated the beneficial effects of probiotic microorganisms, using different formulae and with numerous purposes of preventing or treating diseases. |
Objective
To evaluate the anti-diarrhoeal activity of Bacillus coagulans MTCC 5856 (LactoSpore®) strain along with its effects on intestinal motility in Wistar rats.
Study Design
Anti-diarrhoeal Activity
- The study involved a total of 36 male Wistar rats (weighing 150–200 g), which were equally divided into 6 groups (n=6)
- Animals were under fasting for 18 h before being randomized into different treatment groups as follows;
- Group 1: Normal Control (no treatment)
- Group 2: Positive Control (Loperamide, 1 mg/kg)
- Group 3: Negative Control (Maltodextrin)
- Animals from group 2 to 6 were treated with castor oil (1 ml/animal) orally to induce diarrhoea
- During the study, animals had access to water only
- Post castor oil challenge, animals were observed for defecation at 4 h, 8 h and 12 h time point
- Weights of the faecal material were measured and quantification of water consumption over 12 h periods was also performed
Gastrointestinal Motility Test
- In this study, 30 Wistar rats were fasted for 18 h and were randomly divided into 5 groups (n=6) as follows;
- Group 1: Normal Control (2% gum acacia, oral)
- Group 2: Positive Control (1 mg/kg Atropine sulphate, intraperitoneal)
- Group 3, 4 and 5: LactoSpore® (40, 80 and 160×106 cfu/kg, respectively)
- Deactivated charcoal (3%) in aqueous gum acacia (2%) was administered orally to animals from group 1, 3, 4 and 5 post 4 h of the treatment, whereas group 2 was treated post 2 h of the treatment
- Using CO2 asphyxiation method, animals were euthanized post 1 h of charcoal meal administration
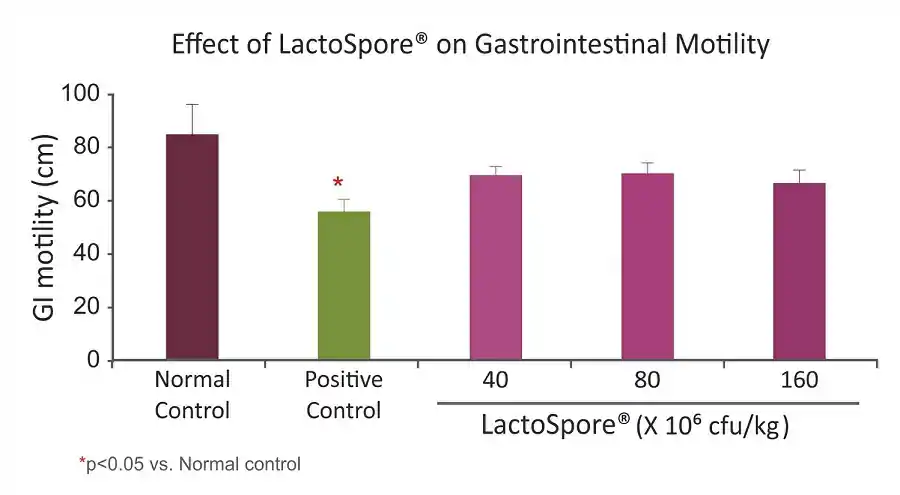
- Gastrointestinal motility was measured by calculating the distance covered by the charcoal meal in the intestine (i.e. from the pylorus to the caecum)
Results and Discussion
To evaluate the safety and tolerability of Bacillus coagulans (LactoSpore®) at a dose of 2×109 cfu (spores)/day in healthy adults over a 30-day supplementation period.
- Faecal weight was decreased in the groups treated with LactoSpore® in a dose-dependent manner with highest dose (160×106 cfu/kg) showing significant decrease similar with Loperamide (p<0.05)
- Similar results were observed with gastrointestinal motility test, where in LactoSpore® at 160×106 cfu/kg dose exhibited almost similar effects to that of Atropine sulphate, a standard drug
Conclusion
LactoSpore® exhibited significant anti-diarrhoeal activity and inhibited the gastrointestinal motility. Hence, it could be considered for further evaluation in the management of diarrhoea.
Physiological effects of a combination of Cinnulin®* with probiotics
Am J Immunol. 2013;9(4):103–09
Probiotics have been the integral part of human diet since long time, wherein they were regularly consumed as a part of fermented foods with specially added active live cultures, such as in yogurt and soy yogurt. However, their popularity is on the rise since past couple of decades as dietary supplements, owing to their wide range of biological benefits. Probiotics have been known to be beneficial for certain health conditions ranging from diarrhoea to urinary tract infections to diabetes and cardiovascular diseases. They also play an important role as an immune-modulator and help impeding allergic and inflammatory responses by stabilizing the immune system. Supplementation of diet with fermented dairy products, including the use of probiotics, especially of Lactobacillus and Bifidobacterium genera in combination with other natural compounds has shown strong synergetic effects, such as immune-modulation, regulation of cholesterol and blood sugar levels. |
Objective
To evaluate the synergistic effect of combination of cinnamon extract (Cinnulin®) and LactoSpore® on immune system and other physiological functions
Study Design
Phagocytosis
Phagocytic activity was determined by adapoting a model of synthetic microspheres based on 2-hydroxyethyl methacrylate using peripheral blood cells.
Sterptozotocin-induced Hyperglycemia
- Female BALB/c mice (8-week-old) were pretreated with streptozotocin (STZ) (250 mg/kg), 12 days before the start of feeding with rodent diet enhanced with glucan
- Effect of diet supplementation on blood glucose levels in mice with experimentally-induced hyperglycemia was studied on day 7 and 14
- Additionally, influence of diet supplementation on serum levels of cholesterol, triglycerides, LDL and HDL was also evaluated
Fasting Blood Glucose Levels
Here, mice were fasted for 24 h before blood glucose level was measured at different time points.
Diet-induced Hypercholesterolemia
- Animals were fed with cholesterol-rich diet and cholesterol levels measured after 14 days of feeding were used as positive control
- Individual groups were treated for 40 days and cholesterol levels were measured at various time points
Experimentally-induced Colitis
- Dextran Sulfate Sodium (DSS) (3.5%) was used in drinking water for 20 days to induce the colitis
- Extent of colitis was assessed by monitoring body weight, stool consistency and blood in the stool as well as the survival rate
Results and Discussion
- Combination of cinnamon extract and LactoSpore® showed statistically significant phagocytosis at different time points (i.e. day 1, 7 and 14)
- In experimentally-induced hyperglycemia model, combination group showed significant decrease in blood glucose level, whereas cinnamon extract alone did not show any efficacy
- In STZ model, combination treatment resulted in reduced levels of total cholesterol and LDL compared to control group
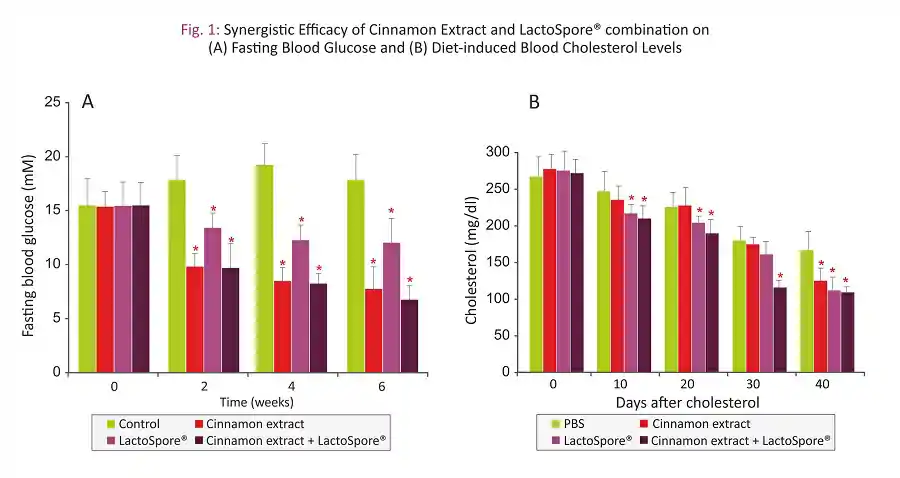
- Data from fasting blood glucose levels suggested that both combination treatment and cinnamon extract alone significantly reduced glucose levels when compared to control group (Fig. 1A)
- Cinnamon extract-LactoSpore® combination showed significantly lowered cholesterol levels at all tested intervals starting from day 10, whereas cinnamon extract alone started showing its effect only after 40 days of the treatment (Fig. 1B)
- Efficacy of the treatment was evaluated by measuring the colon length of mice in experimentally-induced colitis model. Results showed that that both LactoSpore® alone and the combination group significantly decreased the DSS-induced shortening of the gut length. However, the combination group showed colon length virtually identical with control mice (i.e. 10.8 cm)
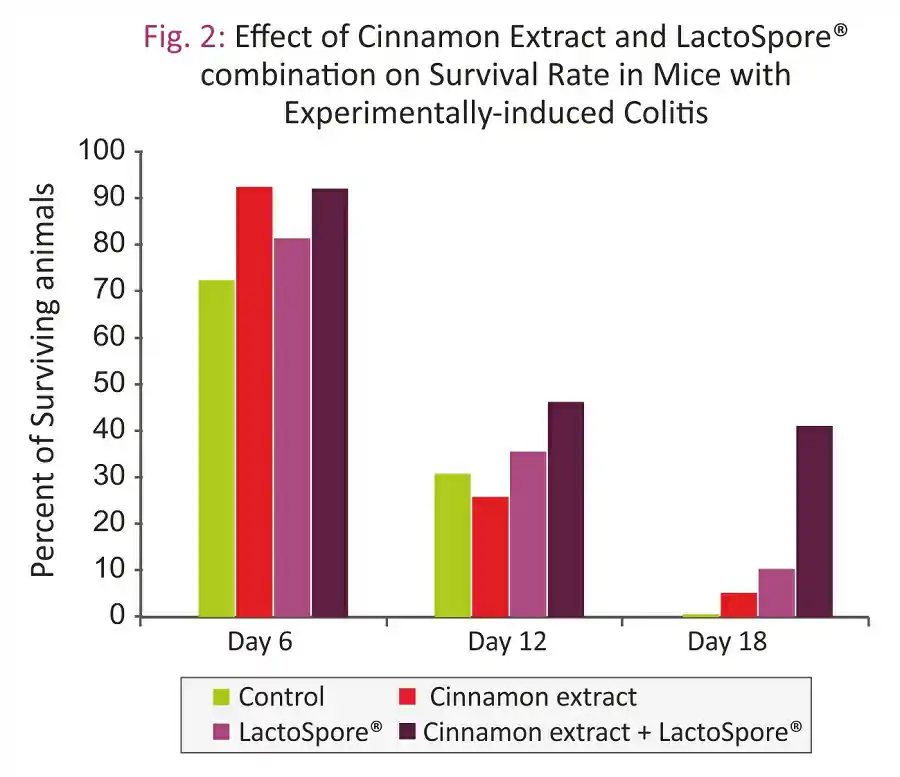
- Furthermore, effect of longer exposure to the lethal dose of DSS on the survival rate revealed that only combination group was able to improve the survival rate (Fig. 2)
Conclusion
Given that cinnamon extract and LactoSpore® combination providing the synergistic effect, it can be a novel and fully natural supplement, which could be helpful in managing overall health and well-being with no possible side effects.
Read More…Note: *Cinnulin® is a registered trademark of Integrity Nutraceuticals, Inc., a Florida corporation.
