A Double-Blind, Placebo-Controlled, Parallel Study Evaluating the Safety of Bacillus coagulans MTCC 5856 in Healthy Individuals
J Clin Toxicol. 2016: 6(1). doi:10.4172/2161-0495.1000283
Different species of both beneficial and pathogenic bacteria are the part of intestinal metabolome. Of which, beneficial bacterial species are responsible for several crucial functions in the intestine; ranging from restraining potentially pathogenic or harmful bacteria, to activating immune responses to aiding proper digestion and absorption of food. Nowadays, homeostasis of gut microflora is greatly influenced by modern lifestyle factors, such as a poor diet, frequent travel and food or water contaminants in combination with increasing age, as well as broad-spectrum antibiotics, which unfortunately kills beneficial bacteria. Hence, probiotics are microorganisms that when administered in adequate amount provide health benefit to the host organism. These microbes are known to induce health benefits by altering the intestinal microecology, producing antimicrobial compounds, and stimulating the body’s immune response. LactoSpore® is one such Bacillus coagulans-based probiotic, well known for its clinical efficacy in several human conditions, including diarrhoea-predominant IBS, improving their quality of life. |
Objective
To evaluate the safety and tolerability of Bacillus coagulans (LactoSpore®) at a dose of 2×109 cfu (spores)/day in healthy adults over a 30-day supplementation period.
Study Design
To evaluate the safety and tolerability of Bacillus coagulans (LactoSpore®) at a dose of 2×109 cfu (spores)/day in healthy adults over a 30-day supplementation period.
- Forty participants were randomized to 1:1 ratio in this double-blinded, placebo-controlled two-arm study
- Participants were divided into 2 groups receiving either tablet containing LactoSpore® (2 billion spores / dose) or placebo for 30 days
- Safety and tolerability of LactoSpore® was assessed over 30 days by safety laboratory parameters (blood haematology and clinical chemistry parameters), anthropometric measures (weight, BMI, blood pressure and heart rate), adverse events, Bristol stool score, tolerability questionnaire and bowel habit diary
Results and Discussion
To evaluate the safety and tolerability of Bacillus coagulans (LactoSpore®) at a dose of 2×109 cfu (spores)/day in healthy adults over a 30-day supplementation period.
- During the 30-day supplementation, all laboratory parameters, anthropometric and vital sign measures were found to be within normal clinical range between both treatment groups
- There was no significant difference between the placebo and LactoSpore® group in terms of the number of bowel movements and the Bristol stool scores (Table 1)
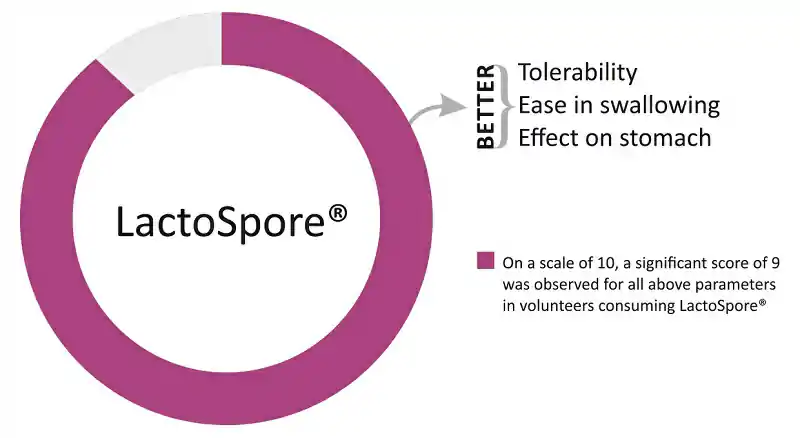
- Subjects from the LactoSpore® group reported that Bacillus coagulans tablets were tolerable and easy to swallow (Fig. 1)
Table 1: Effect of LactoSpore® on Average No. of Bowel Movements and Bristol Stool Scores
| Parameters | Visit | Placebo | LactoSpore® |
|---|---|---|---|
| Average No. of Bowel Movements | Baseline (Day 0) | 1.32±0.42 | 1.19±0.51 |
| End of the Study (Day 30) | 1.14±0.45 | 1.19±0.75 | |
| Bristol Stool Scores | Baseline (Day 0) | 3.76±0.63 | 3.91±0.86 |
| End of the Study (Day 30) | 3.61±0.62 | 4.40±0.87 |
Fig. 1: Effect of LactoSpore® on Tolerability and Other Related Parameters
Conclusion
“Overall, oral administration of LactoSpore® at a dose level of 2×109 cfu (spores) per day for 30 days was safe and well tolerated in healthy subjects.”
Read More…