The effects of Bacillus coagulans MTCC 5856 on functional gas and bloating in adults. A randomized, double-blind, placebo-controlled study
Read moreMedicine (Baltimore). 2023; 102(9): e33109
Objective and Study Design
A randomized, double-blind, placebo-controlled study was conducted on 70 adults for 30 days to assess the efficacy of LactoSpore® in managing functional gas and bloating.
Dosage
The participants were instructed to take LactoSpore® tablets (2 billion spores/day) or placebo once daily in the morning, 30 minutes before food, for 30 days.
Results
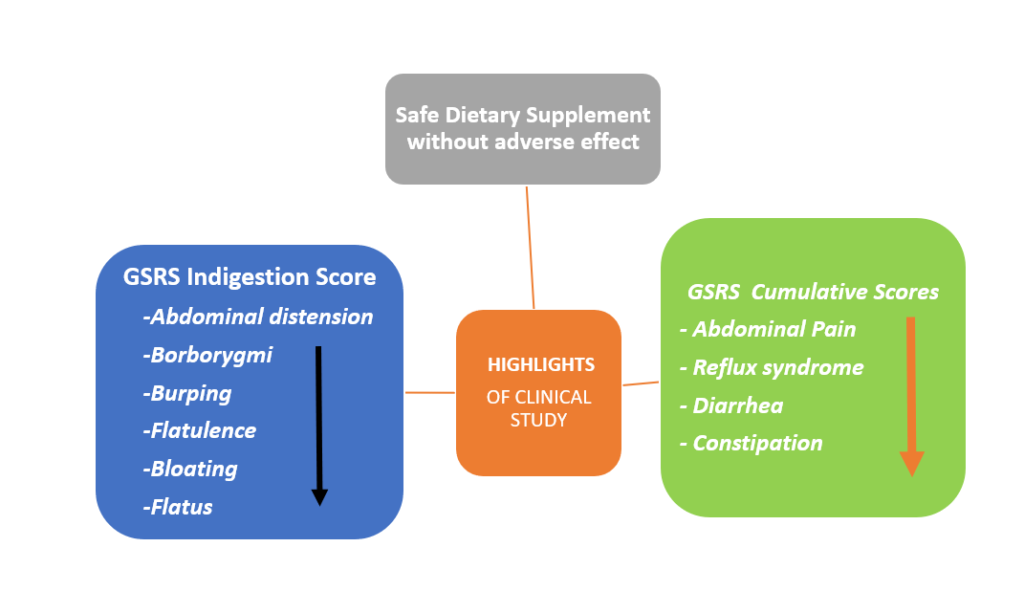
- A significant improvement in the Gastrointestinal symptom rating scale (GSRS) indigestion subscale score, which includes abdominal distension, borborygmi, burping, and flatulence, was observed in the LactoSpore® group compared with placebo after 30 days
- The cumulative GSRS scores for abdominal pain, reflux syndrome, diarrhea, and constipation subscales significantly improved in the LactoSpore® group
- The overall patient score showed a significant improvement in the LactoSpore® group, thus suggesting its beneficial impact on gastrointestinal symptoms
- Moreover, LactoSpore® was found to be safe with no adverse events reported throughout the study period
Conclusion
LactoSpore® (Bacillus coagulans MTCC 5856) supplementation may be an effective and safe approach to reduce the gastrointestinal symptoms in adults with abdominal gas and distension.
Probiotic modulation of gut microbiota by Bacillus coagulans MTCC 5856 in healthy subjects – A randomized, double-blind, placebo-control study
Read moreMedicine (Baltimore). 2023; 102(20): e33751
Objective and Study design
A randomized, double-blind, placebo-control study was designed to assess the impact and safety of LactoSpore® supplementation for 28 days on microbiota composition in 30 healthy adult participants (Age: 25-55 years).
Dosage
The participants were randomly assigned to receive either 1 capsule of LactoSpore®( B. coagulans MTCC 5856) or placebo orally once daily after dinner for 28 days.
Results
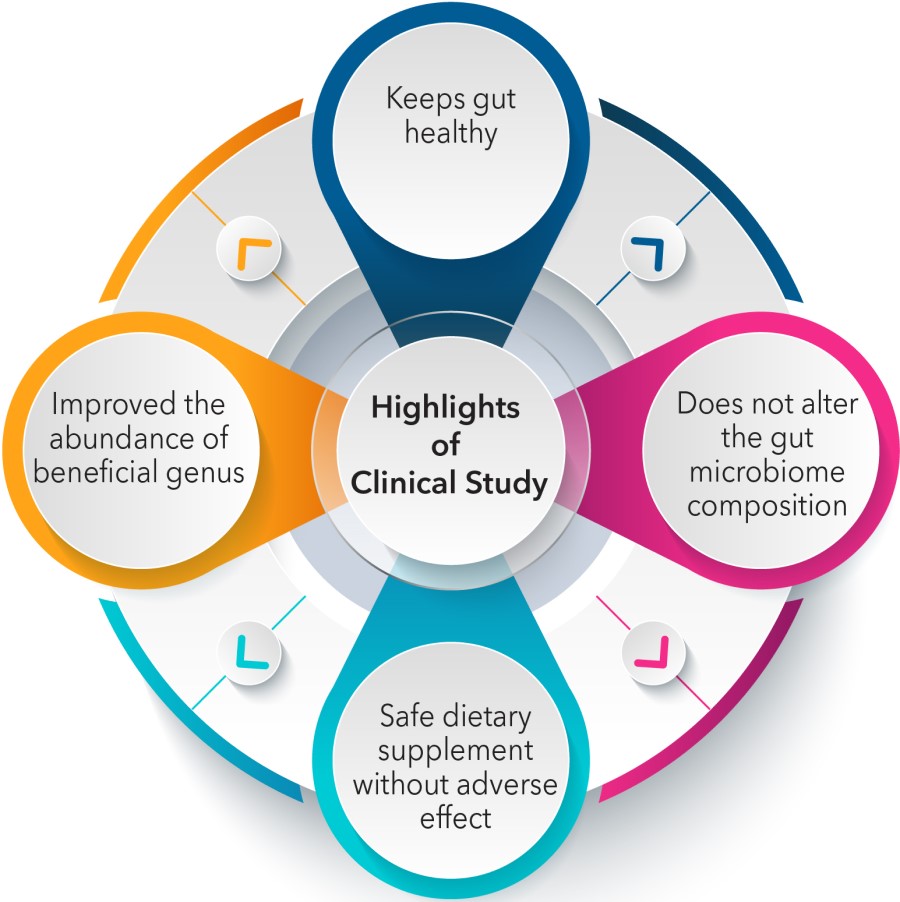
- LactoSpore® supplementation is a safe probiotic and maintains gut and general health parameters in the normal range
- Metagenomics analysis revealed no major changes in the gut microbiome composition in subjects supplemented with LactoSpore® in the bacteriodetes and Firmicutes ratio. Minor decrease in Proteobacteria and increase in Actinobacteria was observed suggesting a positive change in gut microflora
- In addition, an increase in the relative abundance of beneficial bacteria like Prevotella, Faecalibacterium, Blautia, Megasphaera, and Ruminococcus was observed in the LactoSpore® supplemented individuals
Conclusion
LactoSpore® supplementation maintains gut health and general health parameters and does not alter the gut microbiome of healthy volunteers. Minor changes in a few bacterial species may have a beneficial outcome in healthy volunteers.
Bacillus coagulans MTCC 5856 for the management of major depression with irritable bowel syndrome: a randomised, double-blind, placebo controlled, multi-centre, pilot clinical study
Food Nutr Res. 2018;4:60.
Irritable bowel syndrome (IBS) is a common disorder that affects the large intestine. Signs and symptoms include cramping, abdominal pain, bloating, gas, and diarrhoea or constipation, or both. The strong relationship between psychological distress and gastrointestinal symptoms have been reported earlier and even most of the patients suffering from irritable bowel syndrome identify stress and anxiety as symptom aggravators. It is a well-established fact that probiotics improve overall gut health through various means. Probiotics have been shown to improve feeling of wellbeing through influencing the gut–brain axis. Thus, in various disorders, such as irritable bowel syndrome, where depression is comorbidity or aggravator, the modification of microbial ecology in human gut by supplementing probiotics may be an alternative strategy to ameliorate or prevent depression. |
Objective
To assess the safety and efficacy of the probiotic strain Bacillus coagulans MTCC 5856 for major depressive disorder (MDD) in irritable bowel syndrome (IBS) patients.
Study Design
The study was a randomised, double blind, placebo controlled, multi-centre, pilot clinical study in irritable bowel syndrome subjects, suffering with severe depression symptoms.
- Patients (n = 40) diagnosed for MDD with IBS were randomized (1:1) to receive placebo or B. coagulans MTCC 5856 at a daily dose of 2 × 109 cfu (2 billion spores) for 90 days
- Changes from baseline in clinical symptoms of MDD and IBS were evaluated through Hamilton Rating Scale for Depression (HAM-D), Montgomery-Asberg Depression Rating Scale (MADRS), Center for Epidemiological Studies Depression Scale (CES-D) and Irritable bowel syndrome quality of life questionnaire (IBS-QOL). Secondary efficacy measures i.e. Clinical Global Impression-Improvement rating Scale (CGI-I), Clinical Global Impression Severity rating Scale (CGI-S), Gastrointestinal Discomfort Questionnaire (GI-DQ) and Modified Epworth Sleepiness Scale (mESS)
Results and Discussion
- Supplementation of the B. coagulans MTCC 5856 resulted in significant favourable change (p = 0.01) in HAM-D, MADRS, CES-D, IBS-QOL, CGI-I, CGI-S, GI-DQ and mESS in B. coagulans MTCC 5856 group compared to placebo group
- Serum myeloperoxidase, an inflammatory biomarker was also significantly reduced (p < 0.01) when compared with the baseline at the end of the study
- All the safety parameters remained well within the normal clinical range and had no clinically significant difference between the screening and at the end of the study
Conclusion
B. coagulans MTCC 5856 showed robust efficacy for the treatment of patients experiencing IBS symptoms with major depressive disorder. These findings support B. coagulans MTCC 5856 as an important new treatment option for major depressive disorder in IBS patients.
For information: https://doi.org/10.29219/fnr.v62.1218Bacillus coagulans MTCC 5856 supplementation in the management of diarrhea predominant Irritable Bowel Syndrome: A double blind randomized placebo controlled pilot clinical study
Nutr J. 2016;15:21. doi: 10.1186/s12937-016-0140-6
Several health benefits are known to be associated with probiotics consumption, including immune-boosting, protection against diarrheal diseases, nosocomial and respiratory tract infections, cholesterol lowering effects, attenuation of overt immune-inflammatory disorders and anti-cancer activities. Lactobacillus and Bifidobacterium are the genera that most probiotic microorganisms belong to; however, some bacteria and yeast strains are also known to possess probiotic properties. Due to the awareness about the benefits of probiotics in gut health and disease prevention and therapy, commercial interest in functional foods containing probiotics strains is gaining importance. As a result, preliminary research has suggested that some strain specific probiotics are possibly helpful in treating various forms of gastroenteritis. |
Objective
To evaluate the safety and efficacy of Bacillus coagulans MTCC 5856 (LactoSpore®), as a dietary supplement, in patients receiving standard care of treatment for diarrhoea-predominant irritable bowel syndrome (IBS).
Study Design
- It was a randomized, double-blind, parallel group, placebo-controlled, multi-centered study involved 36 subjects
- Each subject was randomized in a 1:1 ratio and asked to self administer one tablet of LactoSpore® (each tablet containing 2×109 cfu) as a dietary supplement in addition to either standard treatment or placebo for a period of 90 days
- A gap of at least 4h between the study product (placebo/active) and standard care of treatment was maintained
- Primary end point was evaluation of clinical symptoms of IBS, whereas secondary efficacy measures involved Physician’s global assessment and IBS quality of life, and both were measured through questionnaires
- Visual analog scale (VAS) was used to evaluate abdominal pain
Results and Discussion
- During the study period, both groups showed normal laboratory parameters, anthropometric and vital signs, within the normal clinical range
- Participants receiving LactoSpore® showed a significant decrease (p<0.01) in the clinical symptoms, such as bloating, vomiting, diarrhoea, abdominal pain and stool frequency compared to placebo group (Table 1)
- Additionally, LactoSpore® group showed decreased disease severity (i.e. Physician’s global assessment) and better IBS-quality of life compared to placebo group (Fig. 1A and 1B, respectively)
Table 1: Effect of LactoSpore® on various Clinical Symptoms
| Parameters | Visit | Placebo | LactoSpore® | Significance (p value) |
|---|---|---|---|---|
| Bloating | Baseline | 5.31±0.82 | 5.88±0.93 | 0.1372 |
| Final Visit | 5.93±0.21 | 3.42±0.31 | 0.0037* | |
| Vomiting | Baseline | 4.88±0.93 | 4.02±0.55 | 0.1126 |
| Final Visit | 4.42±0.36 | 2.13±0.19 | 0.0013* | |
| Diarrhoea | Baseline | 5.79±0.95 | 5.70±0.76 | 0.1684 |
| Final Visit | 5.93±0.54 | 3.25±0.42 | 0.0026* | |
| Abdominal Pain | Baseline | 5.64±0.77 | 5.71±0.11 | 0.1539 |
| Final Visit | 5.93±0.21 | 1.82±0.49 | 0.0001* | |
| Stool Frequency | Baseline | 5.41±0.68 | 5.67±0.58 | 0.1485 |
| Final Visit | 5.75±0.11 | 3.11±0.27 | 0.0031* |
*Statistically significant
Table 1: Effect of LactoSpore® on various Clinical Symptoms


Conclusion
Overall, LactoSpore® was found to be safe for human consumption as a dietary supplement and also demonstrated significant efficacy in the management of diarrhoea-predominant IBS.
Read More…Changes in anthropometric measurements, body composition, blood pressure, lipid profile, and testosterone in patients participating in a low-energy dietary intervention
J Chiropr Med. 2013;12:3–14
According to the surveys by National Health and Nutrition Examination between 1971–75 and 2005–06, incidence of obesity has increased by 2-fold (16.6% vs. 36.5%) in women and 3-fold (11.9 vs. 33.4%) in men. Several systematic reviews and meta-analyses have suggested that metabolic risk factors can be reduced by adapting dietary modifications, such as low-carbohydrate foods or low-fat diets. Hence, diets containing low calories and/or caloric restriction play a critical role in the weight management programs, regardless of dietary macronutrients. In addition to dietary modifications, theories suggest that nutritional supplementation with probiotics and probiotics could be another component of weight management programs. Because, it is believed that probiotics and probiotics have significant health benefits on lipid metabolism, mineral absorption and immune function by virtue of their beneficial influences on microbial ecology of the gut. As a result, probiotic- and probiotic-rich nutritional supplementations are being promoted as adjunct for the weight management and health. |
Objective
To evaluate changes in anthropometric measurements, body composition, blood pressure, lipid profile and testosterone following a low energy-density dietary intervention plus regimented supplementation program, including intake of LactoSpore® tablets.
Study Design
- In this 21-day weight loss program, a pre-post intervention design was followed
- The study comprised of the faculty, staff, students and community members from a chiropractic college as volunteers
- All participants received freshly prepared meals (mostly vegan) comprising breakfast, lunch and dinner that included 1200 to 1400 daily calories for the women (n=36) and 1600 to 1800 daily calories for the men (n=13)
- The regimented supplementation program was divided into 3 weekly phases: Reclaim, Release and Restore
Results and Discussion
- Data suggested a clinically significant pre-post intervention decrease in anthropometric measurements [e.g. body weight (−8.7 ± 5.54 lb; −3.9 ± 2.5 kg), BMI (−1.4 ± 0.81 kg/m2), waist circumference (−1.5 ± 1.14 in), hip circumference (−1.2 ± 1.24 in), waist-to-hip ratio (−0.01 ± 0.033)]
- Pre-post intervention decrease in levels of total cholesterol (−30.0 ± 29.77 mg/dL), LDL cholesterol (−21.0 ± 25.20 mg/dL) and triglycerides (−10.1 ± 34.66 mg/dL) was also observed
- A significant change in fat mass (−5.2 ± 4.12 lb; −2.4 ± 1.9 kg) and fat-free mass (−4.0 ± 4.16 lb; −1.8 ± 1.9 kg) was witnessed during pre-post intervention program
Conclusion
Overall, results from this observational study indicated that a low calorie-density dietary intervention plus regimented supplementation program along with patient education improved weight status and lipid profiles.
Read More…